A Buffer Solution Comprises Which of the Following
The reaction between HNO₃. Aweak acid and its conjugate base in solution Oc Aweak acid in solution O d.

Topical And Transdermal Delivery Of L Carnitine Pubmed Ncbi Hypothyroidism Bone And Joint Heart Disease
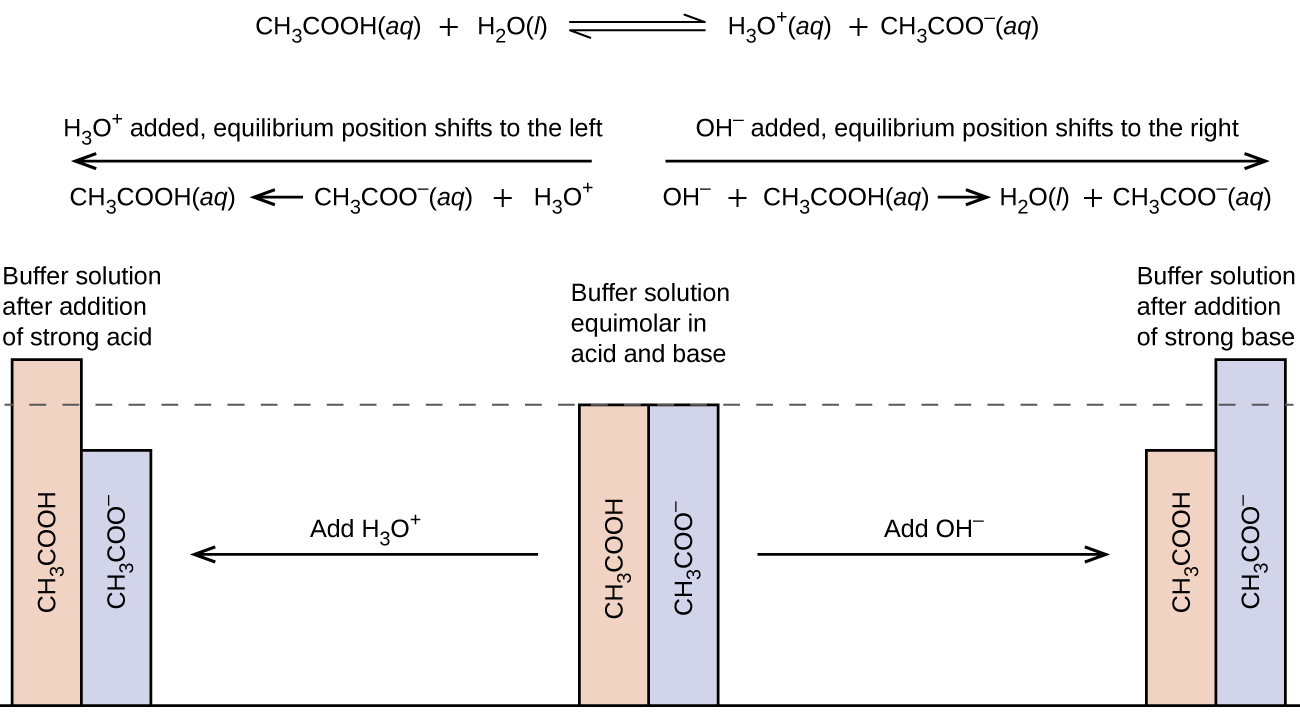
The buffer solution is a solution able to maintain its Hydrogen ion concentration pH with only minor changes on the dilution or addition of a small amount of either acid or base.

. 2Calculate the pH when 288 mL of 0035 M HCl is added to 1000 mL of the above buffer. Aweak base jn solution O e. At what pH does the solution buffer.
A buffer solution is an aqueous solution of weak acid and its conjugate baseIt can also be a mixture of weak base and its conjugate base. Calculate the initial pH of this solution. Buffer solutions are obtained when a weak acid is mixed with its conjugate base or a weak base is mixed with its conjugate acid.
A buffered solution resists a change in pH. Astrong acid in solution Ob. A buffer has a pH of 485 and contains formic acid and potassium formate.
A buffer solution contains t what pH don contains. 1A buffer solution that is 010 M sodium acetate and 020 M acetic acid is prepared. Hence solution of acetic acid and sodium acetate is a Buffers solution.
Acid and its conjugate base in c A weak base in solution pka of ethanoic acid is 474 14. A more technical way of saying this is that a buffer solution consists of a mixture of a weak acid and its conjugate base OR a weak base and its conjugate acid. The p H value of a basic buffer is always greater than 7.
What can you conclude about the concentrations of the components of the. At what pH does the solution buffer. Welcome to Sarthaks eConnect.
As usual report pH to 2 decimal places. A solution that resists significant changes in pH upon addition of a large amount of a strong acid or base. A mixture of a weak acid and its conjugate base or a mixture of a weak base and its conjugate acid is called a buffer solution or a bufferBuffer solutions resist a change in pH when small amounts of a strong acid or a strong base are added A solution of acetic acid and sodium acetate CH 3 COOH CH 3 COONa is an example of a buffer that consists of a weak acid and.
The Ka for CH3COOH is 18 x 10-5 M. Explanation- A buffer solution is a. A buffer comprises of either.
The definition of a buffer is. Weak acid conjugate base Acidic buffer Weak base conjugate acid Basic buffer By producing alternative components this mixture preserves pH homeostasis in the face of any pH shift. A strong acid in solution P Flag question O c.
DA weak acid and its conjugate base in solution. Multiple choice questions MCQs 1A buffer solution comprises which of the following. Astrong acid and its conjugate base in solution.
A unique platform where students can interact with teachersexpertsstudents to get solutions to their queries. A solution that doesnt resist significant changes in pH upon addition of a small amount of a strong acid or base. A Buffer solution contains 036 M sodium acetate CH3 COONA And 045 M acetic acid CH3 COOH pKa48 what is the pH of this buffer solution.
A weak base in solution O d. A weak base and. Maximum buffer capacity.
154 A buffer solution comprises which of the following. AA weak acid in solution. Answer- The correct option is d.
The maximum buffer capacity is achieved when pH pKa or in equivalent terms where H3O Ka. Science Biochemistry QA Library A buffer solution comprises which of the following. This can be accomplished by using a solution containing which of the following.
A buffer is a mixture of a solution a weak acid and its conjugate base or vice versa. BA strong acid in solution. Among the given options N H 4 O H N H 4 C l is the only mixture which consists of a mixture of a weak base and its conjugated salt and thus has a p H greater than 7.
Students upto class 102 preparing for All Government Exams CBSE Board Exam ICSE Board Exam State Board Exam JEE MainsAdvance and NEET can ask questions from any subject and get quick answers by. A student is asked to prepare a buffer solution with a pH of 400. HClO NaOH NaClO.
A buffer comprises Solution a A weak acid in solution b A strong acid in solution comprises which of the following. In the given options only option-A has weak acid C H 3 C O O H and its conjugate base C H 3 C O O N a. A weak acid in solution O b.
A buffer solution comprises which of the following a. Chemistry questions and answers. A weak base in solution D.
View the full answer. A buffer is defined as a substance which is able to resist changes in pH of a solutionIt usually comprises of the mixture of a weak acid with its conjugate base or a weak base with its conjugate acid. Question 5 A buffer solution comprises which of the following.
A solution made by mixing 100 mL of 0100 M HClO and 50 mL of 0100 M HCl IS NOT a buffer. Not yet answered Marked out of 150 O a. A weak acid and its conjugate base in solution 4.
A buffer solution can be made by mixing a weak acid with one of its salts OR mixing a weak base with one of its salts. The pKa of ethanoic acid is 474. A A weak acid in solution b A strong acid in solution c A weak base in solution d A weak acid and its conjugate base in solution.
A strong acid in solution C. Thus a solution by mixing 100 mL of 0100 M HClO and 50 mL of 0100 M NaOH IS a buffer because. CA weak base in solution.
Here weak acid is HClO and its conjugate base is NaClO. A buffer solution contains ethanoic acid and its conjugate base. Buffer Solutions are used in fermentation food preservatives drug delivery electroplating printing the activity of enzymes blood oxygen carrying capacity need.
There are no single components in it such as only weak acid strong acid or weak base. A Weak acid in solution B. 3 A Buffer solution comprises which of the following.
Buffer solution This is a mixture that minimises pH changes on addition of small amounts of acid or base A weak acid is used and its conjugate base formed by adding weak acid salt or neutralising the acid by adding aqueous alkali.

Now Available On Our Store Continuum Reef Check It Out Here Http Www Freshnmarine Com Products Continuum Reef Potassium Supplements Sps Coral Potassium

Pin On Eye Vision Ear Problems

Pin On Eye Vision Ear Problems

Modern Mother S Day Digital Signage Menuboard Design Digital Signage Digital Menu Boards Digital Menu

Bathtub Seats Iroonie Com Bathtub Seat Modern Restaurant Seating

Icoled 2017 Lamp 3d Printing Novelty Lamp

What Is A Buffer And How Does It Work Westlab

Pin On Eye Vision Ear Problems

A Buffer Solution Comprises Which Of The Following A A Weak Acid In Solution B A Strong Acid In Brainly In

Curved Led Screens Tasarim Alanlar






Comments
Post a Comment